Week 9: The Final Piece of the Puzzle
Aditya L -
Hey everyone,
Thanks for tuning in. Over the past week, I was able to find one of the critical interactions and lend some clarity to the work that I’ve been doing for the past few months now.
But first, heres a brief recap of last week: When there is an empty rhodopsin with no molecules in a solution with both Benzo[A]Pyrene (environmental pollutant) and 11-cis-retinal (the natural human molecule), the Benzo[A]Pyrene first docks to the dark state Rhodopsin, but is prematurely released. This causes the 11-cis-retinal to be expressed despite Benzo[A]Pyrene’s better binding affinity.
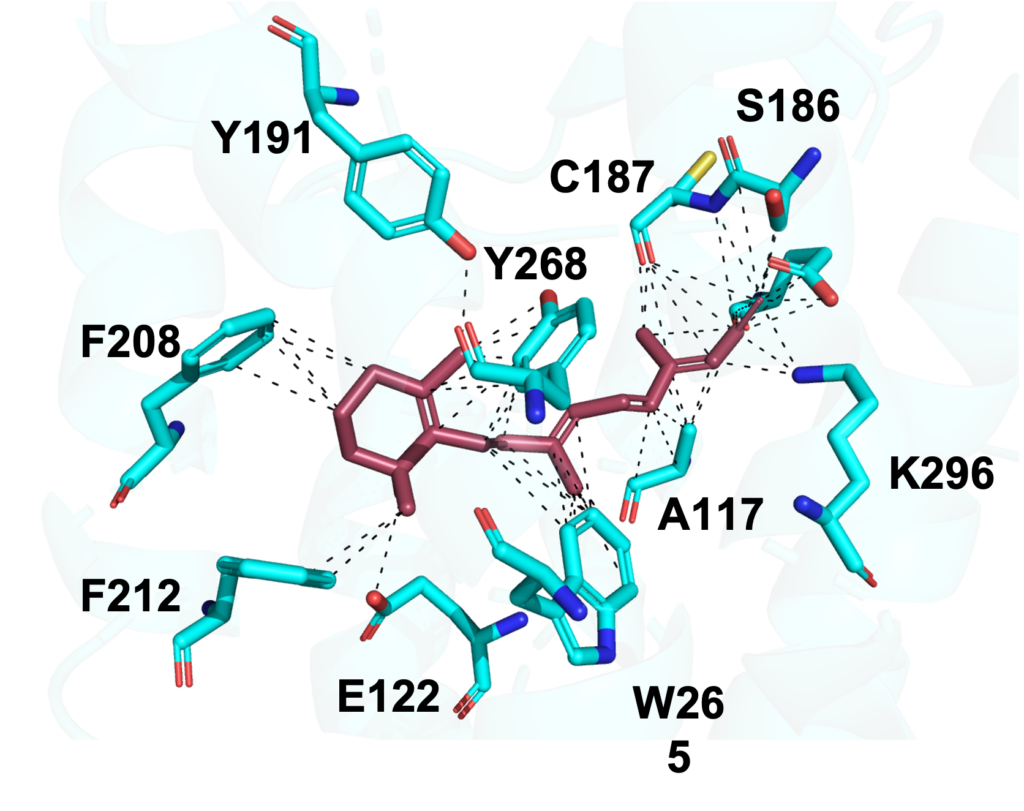
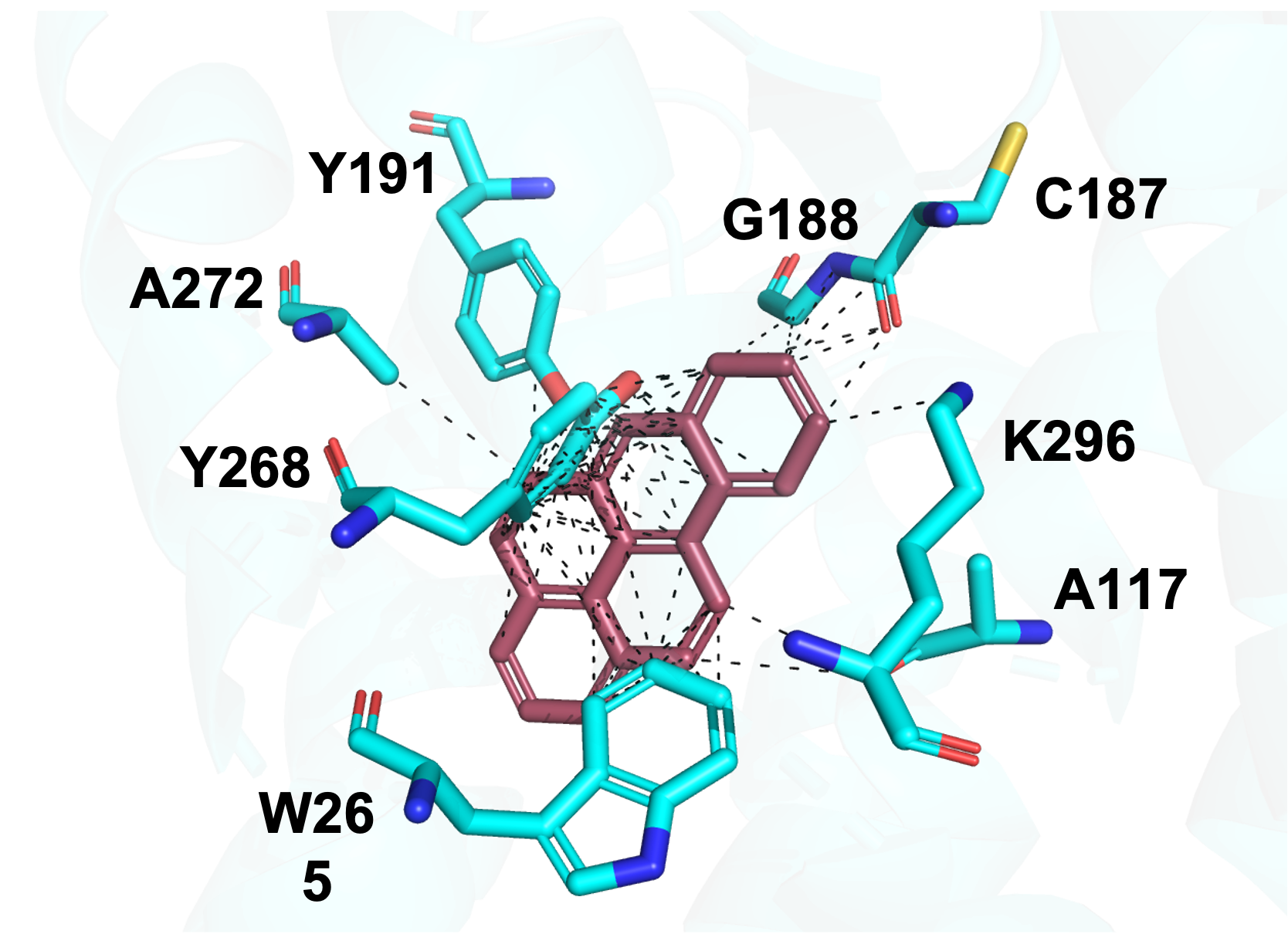
I started off this week by creating some residue figures (which basically show a zoomed in interaction of the protein). Interestingly, although Fig 2. for Benzo[A]Pyrene shows more interactions (easier to see in a 3D view), it doesn’t form a Schiff-base linkage with the K296 residue. A Schiff-base linkage is basically a strong attachment interaction which leads to a protonated state, that is ultimately stabilized by some counterion in the protein. Because 11-cis-retinal (Fig. 1) has an aldehyde at the very end, it is able to form a Schiff-base linkage with the Rhodopsin protein’s K296 residue, which means that after the process has concluded, there is also a counterion (113) which stabilizes the 11-cis-retinal and holds it in place. This is why the 11-cis-retinal does not prematurely detach during photo-transduction. On the other hand, despite adopting a stronger binding affinity, B[A]P only contains carbons and is incapable of forming a Schiff-base linkage with K296, so it does not get protonated, and is not able to hold onto any counterions. Biochemically, this means that the B[A]P’s affinity can only get it to interact the dark state, but will almost never naturally be held in the light-state conformation due to premature release.
Now that I have found this interaction, I will be focussing more on the implications next week, as well as a formal recap of my project as a whole.
Sincerely,
Aditya Lakshminarasimhan
A.

Comments:
All viewpoints are welcome but profane, threatening, disrespectful, or harassing comments will not be tolerated and are subject to moderation up to, and including, full deletion.