Week 4: The Purification Process
Rabia H -
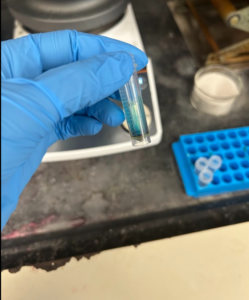
Hello everyone! Welcome back to week 4 of my senior project. Last week, I left off needing to purify the first dye I made. My lab monitor tried a purification method on my dye over the weekend, but it wasn’t enough. I would later purify it on Wednesday and Friday. When I entered the lab on Monday, my dye looked like this:
It’s quite blue and quite different from the dark red from last week. That’s because my lab monitor removed some impurities from the dye that made it red. Also, since Dr. Usama’s lab works with Cyanine-7 dyes, they tend to be blue or green as they absorb light around 700 nm in the near-infrared spectrum (NIR). However, looking at a NIR spectrum graph, you’ll realize that around 700nm is red.
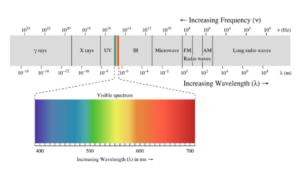
The Cy-7 dyes absorb the wavelengths of the color red, and he wavelengths they don’t absorb are what we see, which is the complementary color blue. For example, think about leaves! They look green because they absorb their complementary colors of blues and reds.
Like I said, I didn’t purify my dye that day. Instead, I tried to precipitate another variation of a Zincke salt I made last week. I followed the steps I outlined in the previous blog, but didn’t realize that my LC/MS data didn’t show good results. Reading the mass spectrometer, it picked up the Zincke salt’s mass, meaning some of my desired product had formed. However, I disregarded the rest of the peaks that detected the starting material, and those peaks were high. This meant only a little Zincke salt was formed since a large amount of starting material was left over. Since a small amount of the product was formed, it would be hard to form a precipitate.
Yet, I went on and dissolved the rest of the Zincke salt into the solvent, ethyl acetate, and let it stir. After a few minutes, Dr. Usama came by and examined my work and questioned why a precipitate hadn’t formed, to which I showed him my LC/MS data and realized my mistake. He told me to recover the salt by purging the solution with nitrogen gas, which would dry the solvent. Despite this setback, I learned what to look for on the LC/MS.
On Wednesday and Friday, my lab monitor and I continued to purify my dye multiple times. We used the Preparative HPLC (Prep HPLC) to purify the dye. This instrument is very similar to the LC/MS, in which it separates a solution through liquid chromatography. However, with this, we can collect the waste it produces (the separated solution). Using the Prep HPLC is more involved, as I had to hold a small tube in a flask as the solution ran, waiting to collect the separated dyes in test tubes.
There are two ways to tell whether the waste is a dye or a regular impurity: color and the absorbance spectrum. Since Cy-3, Cy-5, and Cy-7 absorb different wavelengths, reading the peaks from a specific wavelength region lets me know in advance that I need to collect a specific dye. Similarly, since each dye absorbs different wavelengths, they have different colors.
The pink is most likely a Cy-3 dye, the dark blue is most likely Cy-7, and the slightly light blue could be both Cy-7 and Cy-5. To make sure, we ran a separate absorbance spectrum of both the dark blue and light blue dyes and ran them through the Prep HPLC again to get rid of any Cy-5 dye.
Once we got the purified Cy-7 dye, the last step was to dry the dye. We made a stock solution with my dye and let it run through the GeneVac, a centrifuge used to dry solutions. At the end, my dye looks like this:
That’s where I leave it! I do apologize for a longer blog. I touched on many complicated steps, so let me know if you have any questions, and I’ll try my best to go more in-depth. I hope to see you next week!



Comments:
All viewpoints are welcome but profane, threatening, disrespectful, or harassing comments will not be tolerated and are subject to moderation up to, and including, full deletion.