Week 3: The Salt Needs a Little Shaping… To the Lab!
Rabia H -
Hello all! Welcome back to my third blog. This week was exciting as I made my first dye with the Zincke Salt I synthesized last Friday.
For some context, why did I make another variation of Zincke salt last Friday? This salt will act as an intermediate for a fluorescent dye. Another professor in the same department suggested additional experiments that could support our hypothesis of asymmetrical fluorescent dyes preventing aggregation. There are many explanations for why asymmetrical fluorescent dyes prevent aggregation while symmetrical ones are more prone to aggregate, such as from polarity, intermolecular forces, and more, which are being studied. In Dr. Usama’s lab, an undergraduate student focused on experimenting with asymmetrical dyes and proving how they are successful in preventing the dye from aggregating when in use. Now, we need to prove that the lack of aggregation was solely from the asymmetry of the dye and not from any other variable. The dye I made with this variation of Zincke salt is symmetrical, and its performance could support our hypothesis.
Anyways, on to the fun part. How did I get some salt to become a colorful dye?
There were a couple of things I had to do with the Zincke salt before using it in a reaction. The first thing I had to do on Monday was collect my Zincke salt and run it through the LC/MS. My salt was still in its vial, and it was purged under nitrogen gas. The nitrogen gas will help reduce moisture and preserve the compound. Overall, the LC/MS readings on the Zincke salt looked good, meaning the compound is good to use.
Now I had to purify my salt to get rid of the by-product. To do this, I had to precipitate the Zincke salt. Hopefully, if you’ve taken honors or AP Chemistry, you’ll recall what a precipitate is. A precipitate is formed when two aqueous solutions are mixed to form an insoluble solid. That insoluble solid, or the precipitate, is the pure product we want. Since I haven’t purified a solution before, my lab monitor had to walk me through how to do it. We first dissolved the Zincke salt in diethyl ether, a common and well-dissolving solvent in the lab, and let it stir on a heat plate for around 15 minutes. After it’s dissolved, we poured it equally into 4 centrifuge tubes and let it run in the centrifuge. While it spins in the centrifuge, the mixture separates by mass as the denser molecules move to the bottom of the tube while the less dense molecules remain at the top. After a couple of spins, the precipitate lay at the bottom of the tube. I decanted the rest of the solvent and let it dry overnight.
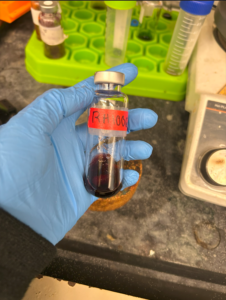
With the Zincke salt now purified, all I need to do is run the reaction to create the dye. Frankly, I was in awe of the dark red color.
I’m glad that I’m able to get the hang of new techniques, each one seeming more complicated than the last. Next week, I’ll purify my dye even further to get rid of the rest of the byproduct (we were supposed to do that Friday, however, plans had to change). I hope you’ll join me there!



Comments:
All viewpoints are welcome but profane, threatening, disrespectful, or harassing comments will not be tolerated and are subject to moderation up to, and including, full deletion.