Week 9: I, too, am Aggregating
Rabia H -
Hello everyone! Welcome to week 9 of my blog. This week, to put it short, plans changed. To keep you all on track, I listed out all my tasks for this week:
- Synthesize and purify 5 mg of my dye (RH1008).
- Synthesize two different isomers of Zincke salts.
- Try a new precipitation method for a Zincke salt.
First, I’ll update you on my progress making 5 mg of dye. I synthesized my dye on Monday and purified it on Wednesday. I repeated the same procedures as usual, but I had issues with the reverse phase ISCO. My dye was supposed to come out around the 10-15 minute mark, but did not. Initially, I thought it was because the method I used was slightly different than the one I used last time; however, my lab monitor believes my dye never formed, or didn’t form a high yield. I was confused as I saw a Cy-7 dye in the UV before I purified it on the ISCO. Yet, the possibility that a minuscule yield of the dye was produced isn’t far off, as the yield of my Zincke salt (starting material) was already low.
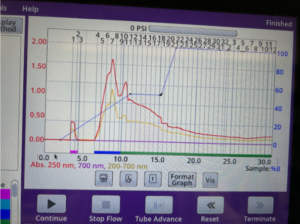
Going forward, I’ll also run my dye on the LC/MS to quantify the final product formed using the mass spectroscopy. Although the UV absorbance detects the presence of the Cy-7 dye, using the LC/MS can provide additional data on the yield with mass spectroscopy.
I won’t lie, I panicked when I realized all my dye was lost. However, I was in a room surrounded by resources and endless chemicals, and there was no excuse to waste them. So, I tried to make my Zincke salts again. Emphasis on “tried” as I quickly found out that I did not have enough of one of my starting materials to make my Zincke salt. Dr. Usama ordered some more, but it won’t arrive until the day before my last day in the lab.
Update TL/DR: I won’t be making 5 mg of RH1008 during my senior project.
I know that sounded disappointing, but on the bright side, I could focus better on other side projects. Dr. Usama asked me to make some more isomers of Zincke salts that I had made once in the first couple of weeks in the lab. He also suggested trying a new method of precipitating my Zincke salt, as it could improve in predicting the yield of the dye. He suggested using dichloromethane (DCM) as a solvent, which is also used for precipitation. For my first salt (RH1010), DCM did not produce a precipitate, so I used diethyl ether instead. The second salt (RH1011) precipitated in DCM incredibly well. I left my pipette in the vial for a couple of seconds, and it already formed a solid.

After I let the mixture stir for a couple of minutes, I transferred the solution to centrifuge vials and ran them in the centrifuge. Once the solvent had evaporated, I wrapped the vial in a Kimwipe and dried it in a vacuum.

Friday was exciting. The perks of working with student lab members are that they’ll try to get you to attend any event possible- and I’m so grateful for that. There was an annual Chemfest at UTSA where students were rewarded for their achievements, presented their works, and even had the chance to dump water on professors. Oh, and there was food. Lots of food. It was a nice break, but also bittersweet as I realized next week would be my last week.
Anyways, that’s where I leave you for this week! I hope you’ll join me for my last blog post next week. I’m hoping for better results!
