Week 8: Lets go Back in Time….
Welcome back to our weekly ClearEndoscope™ and KnoxFog™ blog series! This week, we’re taking a step back—not in progress, but in physics. We’re diving deep into the science of why fogging even happens in the first place. By understanding the role of surface energy and water condensation, we’re uncovering the very principles that guide the creation of our anti-fog coating. Buckle up, it’s time for some molecular throwback.
But first, let’s set the stage with a quick five-question quiz to test your fog-fighting fundamentals.
Week 8 Quiz
What is surface energy?
A. The total energy of an object
B. The energy of water droplets in the air
C. The excess energy at a material’s surface due to unbalanced atomic forces
D. A new type of rechargeable battery
High surface energy surfaces tend to:
A. Repel water molecules
B. Attract water molecules to lower their energy state
C. Spontaneously combust
D. Form 3D water towers
Why do hydrophobic surfaces cause fogging?
A. They absorb all moisture
B. They allow light to pass through clearly
C. They promote the formation of spherical droplets that scatter light
D. They convert fog to mist
What is the ideal contact angle on a super-hydrophilic surface?
A. 180°
B. 45°
C. 90°
D. 0°
What is the purpose of 3LCAA (Three Liquid Contact Angle Analysis)?
A. To clean medical equipment
B. To measure surface temperature
C. To determine a surface’s energy components using known liquid interactions
D. To test water pressure in laparoscopes
(Answers at the bottom—resist the urge to peek!)
Surface Energy: Why It Matters
To understand how and why KnoxFog™ works, we first need to talk about surface energy—a property that determines how water behaves when it lands on a surface. On a molecular level, atoms at a material’s surface are not fully surrounded by other atoms, so they have “extra” energy compared to atoms in the bulk. That’s surface energy.
-
High surface energy = strong intermolecular attraction = water is pulled in and spreads out (hydrophilic behavior).
-
Low surface energy = weak attraction = water beads up and stays separate (hydrophobic behavior).
This is the basis of whether a surface fogs up or stays clear.
Hydrophobic vs. Hydrophilic: The Battle of Fog
Hydrophobic Surfaces
These have low surface energy. Water droplets on them don’t want to stick, so they form nearly spherical sessile droplets. Under the Volmer-Weber thin film condensation model, this leads to uneven droplet formation—a patchy, fogged-up mess that blocks visibility. In other words: not ideal for surgical scopes.
Hydrophilic Surfaces
These surfaces have high surface energy. They attract water, encouraging droplets to flatten and merge into a thin, even film. This minimizes light scattering and preserves clarity. For ClearEndoscope™, we aim to create super-hydrophilic surfaces that form a fully transparent water film under all surgical conditions.
Measuring Surface Energy: 3LCAA and the Young Equation
To engineer surfaces with the perfect energy profile, we use Three Liquid Contact Angle Analysis (3LCAA). Here’s how it works:
-
Three standard liquids (e.g., deionized water, glycerin, α-bromonaphthalene) with known surface energy values are dropped onto the material.
-
The contact angle of each droplet is measured using high-resolution imaging and DROPTM, a tool that calculates angles based on droplet reflections and shapes.
-
From these angles, we compute the Van Oss-Chaudhury-Good (vOCG) components:
-
γLW (Lifshitz–van der Waals forces)
-
γ+ (Electron acceptor)
-
γ− (Electron donor)
-
These feed into the Young Equation to give a total surface energy profile.
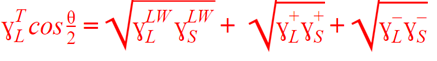
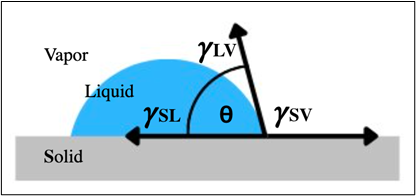
Contact Angles and Triple Points
The contact angle is the angle between the solid surface and the tangent of a droplet at its triple point (where solid, liquid, and vapor meet). This angle tells us how “wet” the surface is:
-
< 90° = hydrophilic
-
> 90° = hydrophobic
-
≈ 0° = super-hydrophilic
Figure 3 (coming soon!) will illustrate how this angle determines fog behavior. For ClearEndoscope™, our goal is always to push contact angles as low as possible to eliminate fog and maintain clear views.
Why This Matters for KnoxFog™
Understanding surface energy lets us tailor KnoxFog™ to flatten water, not fight it. Instead of resisting moisture (which results in fog), we’re building a coating that welcomes water and lays it down in a transparent, light-permitting layer.
By manipulating surface energy, we’re engineering visibility at the atomic level—no small feat in the high-humidity world of surgical endoscopy.
Coming Soon
-
Visuals of contact angles on untreated vs. KnoxFog™-treated lenses
-
Interactive demo of 3LCAA using our lab imaging rig
-
Side-by-side clarity tests in humid chambers with time-lapse fog formation
The science of surface energy might sound theoretical, but it’s the key to crystal-clear surgeries—and we’re bringing it to life, one droplet at a time.
Answers to the Quiz
C (Surface energy is the excess energy at a material’s surface)
B (High-energy surfaces attract water to lower their energy)
C (Fog forms due to spherical droplets on low-energy surfaces)
D (Contact angle = 0° for super-hydrophilic)
C (3LCAA measures surface energy via known liquid contact angles)
Until next time, stay curious—and stay clear.
Cheers,
Shreyash
