Week 3: Rats, Research, and Reality–Brain Surgeries
Shriya S -
Hey everyone! Welcome back to my blog. As mentioned last week, I will be discussing the rat brain surgeries I had the opportunity of experiencing last week. Because scanning and analyzing the IBA1 tissue samples takes longer than I anticipated, I will be going over that next week. I will also be going over GFAP next week, so I don’t overload you all with information in this post, as I feel like have been doing (sorry!).
Brain surgeries
Wow! This was certainly an experience. Last Tuesday, I had the opportunity to witness some brain surgeries done on rats, specifically midline fluid percussion brain injury. This is essentially a controlled way for researchers to give rats traumatic brain injury (TBI). First, researchers do cranial surgery to place an injury hub into rats’ brains. Through this injury hub, as the name suggests, the damage is administered. This cranial surgery involves a craniectomy (removing part of the skull); once a circular part of the skull is removed, the injury hub, which can be compared to a plastic straw but thicker and flatter at the top, is superglued to the surrounding skull. A screw is drilled into the skull for extra stability; it does not penetrate the brain. Throughout this process, researchers are trying to minimize careless damage to the brain for controlled research and, more importantly, ethics. I was not allowed to take pictures of the surgeries, but here is a diagram that shows the careful process of placing an injury hub into the brain of a rat.
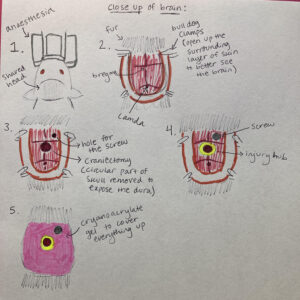
Next, researchers administer the injury itself. As you can see in the diagram above, the injury hub is placed in the middle of the brain–hence, the name midline fluid percussion brain injury. Essentially, there is a hammer attached to a pendulum, and this hammer strikes a cylinder filled with fluid (measures are taken to ensure there are no air bubbles in the cylinder). Once the cylinder is hit, the fluid is injected into a syringe which then injects fluid into the brain of the rat through the injury hub. This builds pressure throughout the brain of the rat, modelling diffuse TBI–TBI that affects the whole brain, instead of a localized part. There is a device attached to this that determines the amount of power the hammer struck the cylinder with, and thus, with how much power the rat was hit with. On Tuesday, they were aiming for 320-340 millivolts, but because of extraneous factors like leaky hubs (where the pressure escapes through gaps between the hub and the skull), they sometimes fell under that range. Also because of the general variability seen in science, some rats could not make it unfortunately, even after CPR. It is here and notably throughout the whole process, the empathy of researchers especially struck me.


I’m very grateful I was able to watch these surgeries to understand the whole process–the pretty and the ugly–from giving rats TBI to getting tissue samples from their brains and eventually analyzing antibody stains on a computer for specific TBI pathology. It also gave me a greater appreciation for the lives of rodents who dedicate their lives to science and how that same science takes careful measures to ensure they are being treated properly (e.g., amount of anesthesia, how their skin should be stapled after surgery, heating pads, various topical anesthesia…). It’s just so amazing.

Comments:
All viewpoints are welcome but profane, threatening, disrespectful, or harassing comments will not be tolerated and are subject to moderation up to, and including, full deletion.