Transfection Mechanism
Hello everyone! This week, I will discuss the transfection mechanism and the protocol we are following.
Transfection is a process that genetically modifies cells. There are two types: transient and stable transfection. Transient transfection involves short-term protein expression achievable through chemical or physical methods. Stable transfection, on the other hand, involves long-term protein expression and can be achieved through biological or physical methods.
In this context, “short-term” means that the reproduced cells will not contain the DNA plasmid added, while “long-term” means that the DNA plasmid will be present in the reproduced cells as well.
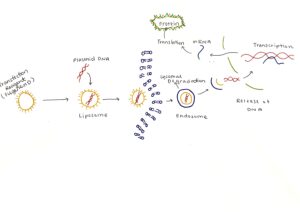
We opted for stable transfection through lipofection, as this allowed us to expand the cell line from a small amount of cells. Lipofection is a process in which the transfection reagent, containing a combination of lipids, forms a lipid lysosome (liposome) around the plasmid DNA harvested from bacteria cells. The DNA has negative phosphate ions in the backbone that attract the cationic lipids through electrostatic interactions. The cationic lipid used in this case is DOTAP.
Once the liposome forms, the cationic lipids interact with the negatively charged membrane to form an endosome. The DOPE lipid in the transfection reagent promotes endosomal escape, allowing the release of the plasmid. Once the plasmid is released into the nucleus, transcription occurs, resulting in the creation of mRNA encoding for the protein. Following this, translation occurs, and the Axl protein is created.
The transfection reagent we used was FuGENE HD. On the first day, we plated and counted the cells using a digital cytometer to ensure equal cell numbers in each well. Following this, we allowed the cells to reach 50-80 percent confluency, where they covered 50-80 percent of the well space. We used Opti-MEM media, which lacks serum, to ensure the cells were not distracted by nutrients in the media and only uptook the liposomes.
On the second day, we replaced the media and added the Axl plasmid DNA along with the transfection reagent to two of the wells. Two other wells were left with new Opti-MEM media as controls.
Three days later, we replaced the media once again. In the next few days, we will add an antibiotic to kill off the cells that did not uptake the Axl plasmid. This antibiotic will also be added to one of the wells set aside as a control and should kill off all the cells in the well.
Additionally, once this process is completed, we will conduct a gene expression assay to ensure the desired protein is expressed in the cells.
Thank you for reading! I will discuss the gene expression assay next week.
