And the winner is…
Last week, our final seven solutions were placed in the In Vitro model, each secured in their own Franz Diffusion Cells. This week, we meticulously collected samples at fixed intervals through the sampling ports via a syringes. After careful analysis, we now have a clear winner.
It is my pleasure to present to you the results from the permeation studies:
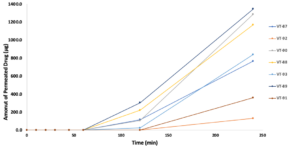
And without further ado— ladies and gentlemen, chemists and curious minds—the moment you’ve all been waiting for is finally here. Please put your hands together for our final winning solution:
VT-89
A perfectly curated blend of:
- 10% Temozolomide (TMZ)
- 50% Miglyol 812 N (Medium Chain Triglyceride)
- 19.5% Propylene Glycol
- 0.5% Alpha-tocopherol
- 10% Labrasol
- 10% Transcutol HP
VT-89 will be going home with the grand prize: its very own nasal spray device, a trophy for the most optimal TMZ spray solution, and an all-inclusive trip to the next phases of testing.
Of course, there is still a long road ahead before VT-89 can become a commercial drug available for sale on the market. This journey includes solution refinement*, animal studies, toxicology evaluations, human trials, and FDA Approval—
not to mention, the significant investment and capitalist component.
*Room for Growth
As much as I’d like to believe so, our solution is not perfect, and needs refinement.
Nasal sprays, by nature, face several limitations. Mucosal thickness, blood flow, nasal congestion all contribute to variability in absorption, reducing permeability. Nasal mucus is constantly being cleared intro the back of the throat at a rate of 5mm/min, minimizing contact time for absorption. Long term use— likely necessary due to the GBM’s resistant nature— can cause nasal irritation. And finally, the nasal cavity itself faces severe volume restraints, only able to hold 25–250 µL per dose.
Future research could tackle these limitations via mucoadhesive or nanoparticle formulations, or advanced delivery devices that allow for targeted delivery, reduced variability, precise dosing, and controlled absorption.
Likewise, our method could be reformed as well. Due to time and resource constraints we eliminated potential inactive pharmaceutical ingredients via an initial screening based off of quantitative characteristics and theoretical behavior. We also had to perform an even more accelerated version of the accelerated stability studies.
FDA approval requires extensive pharmacokinetic testing— measured using ADME: Absorption, Distribution, Metabolism, and Excretion. While our work via the permeability studies tackled a small portion of absorption, further research in that area as well as the additional three avenues is required.
While recognizing the limitations, I still hope that maybe, just maybe, this solution will act as a jumping point for future research.
Final Word
And that’s a wrap, folks.
Ten weeks of reading hundreds of papers, spending countless hours in the lab, and writing research papers, blog pots, and presentations has culminated into the development go one final solution — one that I hope one day will revolutionize treatment for glioblastoma multiform. Hopefully, with a lot more testing and little more hope, we will be able to increase the median post-diagnosis survival time to more than 14.6 months — giving patients a renowned hope and possibility for the future.
Before I end, a few thank you’s are in order. To the people whose efforts made this project possible:
- Dr. Goskonda, my onsite mentor — Thank you for guiding me through every phase of testing, sharing your immense academic and professional, and teaching me so much about pharmacy and chemistry. These are lessons I will carry with me beyond this project.
- Dr. May, my AP Research professor — Thank you for being with us from the very start and carefully guiding us through this process, as we grew from research novices to independent investigators.
- Sullivan — Thank you for all of the work you do behind the scenes to make sure the SRP experience is smooth and fulfilling.
- My Partner, Nneamaka Oniya — my ride our die throughout this project. From venting through roadblocks to peer editing each others papers/ blog posts, and giving the best advice — thank you for it all.
- And finally, to you —my readers — thank you for joining me on this journey full of its ups and downs, for your questions, your comments, and your support through my breakthroughs and failures.
Dear readers, it has been a pleasure to bring you along this journey. I will miss the excitement of sharing my discoveries with you every week (the stress of cramming blog posts on Sunday afternoons though— maybe not so much). While our online chapter has come to an end, I would like to extend an invitation for to join me one last time. I will be presenting my project at BASIS Chandler on Saturday, April 26, 2025. I’d love to see you there!
Thank you, thank you, thank you.
Until next time.
Smilangi signing off!

